Drosophila is able to taste a remarkable diversity of organic molecules. We discovered a family of 60 seven-transmembrane-domain genes, the Gr (Gustatory receptor) genes, and showed that they encoded taste receptors. We are now studying how the receptors, neurons and circuits of the taste system allow the animal to make feeding decisions and oviposition decisions. We also study how the taste system evolves: when a species adapts to a new environment, its taste system must adapt to detect new toxins and exploit new resources.
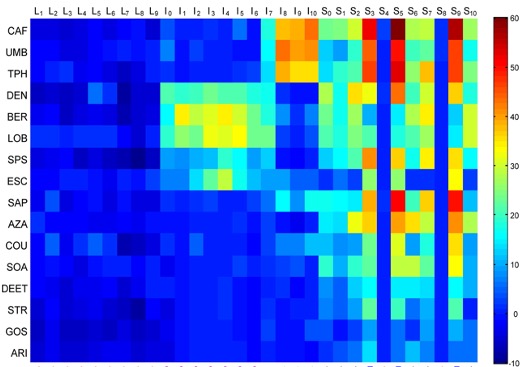
Responses of taste neurons to tastants
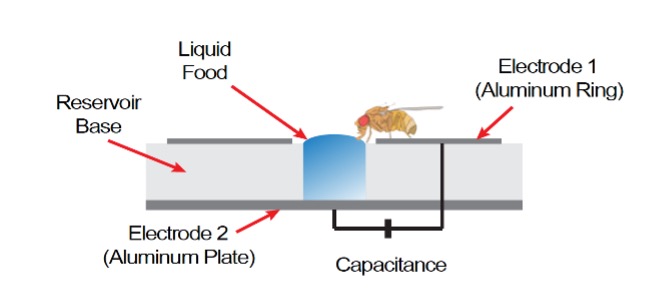
Feeding Assay
We have recently characterized non-canonical mechanisms of taste coding. This work was enabled by a rarely used form of electrophysiology that we call “base-recording”. The method allows measurement of features of taste responses that can not be measured with the conventional “tip-recording” method that has been used nearly universally in the field for the past 50 years.
Using this method, we found that taste neurons of Drosophila are much more sensitive than previously thought. They have a low spontaneous firing frequency that depends on taste receptors. Taste neurons are activated at a short distance by many tested odorants, including DEET. DEET can also inhibit certain taste neurons, revealing that there are two modes of taste response: activation and inhibition. We have characterized electrophysiological OFF responses and found that they link tastant identity to behavior: the magnitude of the OFF response elicited by a tastant correlated with the egg laying behavior it elicited. In summary, the sensitivity and coding capacity of the taste system are much greater than previously known.
We note that 30% of the world’s agricultural output is damaged every year by insects, which make feeding decisions largely on the basis of taste. Thus advances in understanding of taste may have enormous implications for the security of the world’s food supply.
References
Dweck, H.K.M and Carlson, J.R. (2023) Diverse mechanisms of taste coding in Drosophila, Science Advances 9, eadj7032
Wang, W., Dweck, H.K.M., Talross, J.S., Zaidi, A., Gendron, J.M. and Carlson, J.R. (2022) Sugar sensation and mechanosensation in the egg-laying preference shift of Drosophila suzukii,
eLife 11:e81703.
Xiao, S., Baik, L.S., Shang, X., and Carlson, J.R. (2022) Meeting a threat of the Anthropocene: Taste avoidance of metal ions by Drosophila,
PNAS 119, e2204238119.
Dweck, H.K.M., Talross, G.J.S., Luo, Y., Ebrahim, S.A.M., and Carlson (2022), J.R. IR56b is an atypical ionotropic receptor that underlies appetitive salt response in Drosophila,
Current Biology 32: 1776-1787.
Dweck, H.K.M. and Carlson, J.R. (2020) Molecular logic and evolution of bitter taste in Drosophila, Current Biology, 30,17-30.
Joseph, R.M., Sun, J.S., Tam, E., Carlson, J.R. (2017) A receptor and neuron that activate a circuit limiting sucrose consumption, eLife 2017;6:e24992.
Delventhal, R. and Carlson, J. R. (2016) Functions of Drosophila bitter taste receptors in different neuronal contexts, eLife 2016;5:e11181.
Weiss, L., Dahanukar, A., Kwon, J.Y., Banerjee, D. and J. R. Carlson (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258-272.

